Role of CGP in overcoming the roadblocks in Cancer treatment

Dr. Hemanth Kumar
MBBS,MD,DM, Medical Oncologist, Mangalore Institute of Oncology, Mangalore
The case study emphasizes the importance of CGP based on the biomarkers identified in the tumor in turn personalizing the cancer treatment to the patient.
Clinical Presentation
Our proband, a 71-year-old female, was diagnosed with stage IV carcinoma of the sigmoid colon with liver and lung metastasis at 69 years of age. She did not have any family history of cancer. She did not have any habits like smoking, consuming alcohol, or tobacco as well. She was treated with FOLFOX and had a good response to the drug. She was found to be KRAS mutated and was put on Bevacizumab. She developed perforation after two cycles and had to undergo an anterior resection. She was continued on FOLFOX and was seen to have a good partial response after 12 cycles. Further, she was put on Capecitabine maintenance. On follow-up, she was noted to have clinical progression and an increase in CEA levels. She was given 6 cycles of FOLFIRI. PET scans done post-chemotherapy did not show a significant regression in the disease in the primary and metastatic regions. The treating oncologist further wanted to decide on the next line of treatment to deliver the best outcome to the patient.
Considering the roadblock in the treatment course of the patient and to better understand the underlying molecular profile of the patient’s tumor, the clinician opted for the TarGT Indiegene panel. This panel provides a complete and Comprehensive Genomic Profile (CGP) of the patient’s tumor along with immunotherapy markers TMB, MSI, and PD-L1by IHC. TarGT Indiegene report revealed clinically relevant variants p.Gly12Val in KRAS and p.Glu545Lys in PIK3CA genes. Mutations in both PIK3CA and KRAS lead to alterations or dysfunction in two pathways: PI3K/AKT and MAPK pathway respectively. Targeting only one pathway may lead to resistance to therapy, owing to the active state of the other pathway (W ee et al., 2009), hence, a combination of drugs or a drug that may target both pathways may lead to inhibition of tumor growth (Kim A et al., 2013; Poulsen, T. S et al., 2021; Ganesan, Pet al., 2013). Co-mutation of PIK3CA and KRAS gene are found in 5.2% of Colorectal cancer patients (Zhang, J et al., 2015). A clinical study showed that colorectal cancers with KRAS and PIK3CA bi-mutations are commonly coexistent and are more likely to cause liver metastasis (Li, H. T et al., 2011). A few clinical studies suggested that the co-mutation of KRAS and PIK3CA gene is associated with poor prognosis in CRC patients (Luo, Q et al., 2020; Liao, X et al., 2012).
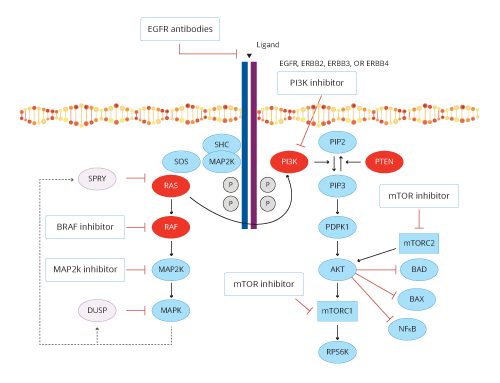
Figure 1 represents the various treatment options in CRC tumors with activated RAS and PI3K pathways (De Roock,Wendy, et al. 2011)
Furthermore, TarGT Indiegene also provided the oncologist with information about the other tumor-agnostic markers. PDL-1was negative, MSI was stable and the TMB was low for this patient.
GENETIC TEST REPORT INTERPRETATION AND DISCUSSION
This case study emphasizes the importance of CGP which broadens the treatment options which are personalized to the patient based on the biomarkers identified in the tumor sample. This proves to be of great help to the oncologist, especially in scenarios where multiple lines of treatment based on the standard of care guidelines, seemed to have failed, thus enabling them to choose the most effective treatment strategy based on the biology of the disease. In this case, for example, though there are no FDA-approved drugs for this presentation, the possibility of targeting downstream biomarkers beyond PIK3CA in this PI3K/AKT/mTOR pathway would be a potential option as per the evidence from clinical studies.
Authors: Divya Uchil, Harita Joshi, Sharmishtha Bhattacharya, Ashwini P, Haritha Myakala
 Kindly fill the form below to download our Liquid Biopsy Portfolio brochure :
Kindly fill the form below to download our Liquid Biopsy Portfolio brochure :